The Idea of Electric Potential
A simple voltaic cell is shown. It consists of the copper plate (known as anode) and a
zinc rod (i.e. cathode) immersed in dilute sulphuric acid (H 2 SO 4 ) contained in a suitable vessel. The
chemical action taking place within the cell causes the electrons to be removed from the copper plate and to be deposited on the zinc rod at the same time. This transfer of electrons is accomplished through
the agency of the diluted H 2 SO 4 which is known as the electrolyte. The result is that the zinc rod becomes
negative due to the deposition of electrons on it and the copper plate becomes positive due to the
removal of electrons from it. The large number of electrons collected on the zinc rod is being attracted
by the anode but is prevented from returning to it by the force set up by the chemical action within the cell.
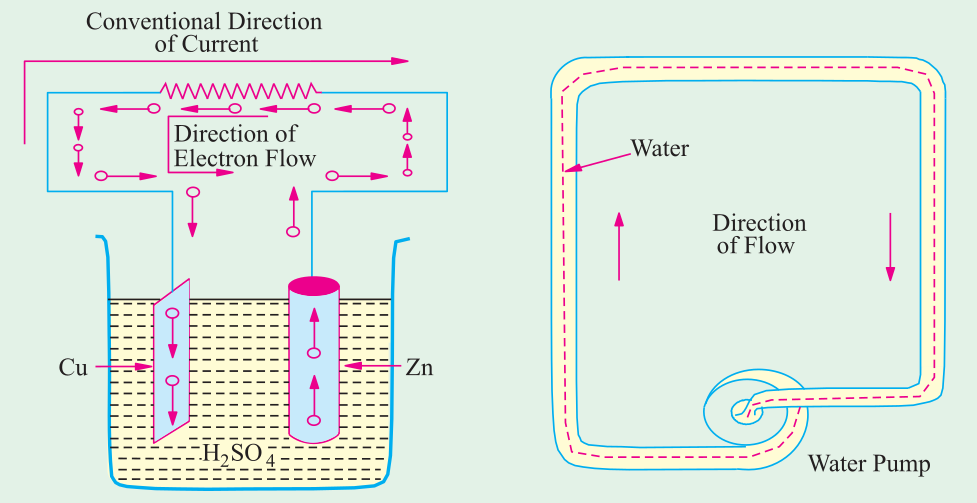
But if the two electrodes are joined by a wire externally, then electrons rush to the anode thereby
equalizing the charges of the two electrodes. However, due to the continuity of chemical action, a
continuous difference in the number of electrons on the two electrodes is maintained which keeps up
a continuous flow of current through the external circuit. The action of an electric cell is similar to
that of a water pump which, while working, maintains a continuous flow of water i.e., water current
through the pipe.
It should be particularly noted that the direction of electronic current is from zinc to copper in
the external circuit. However, the direction of conventional current (which is given by the direction
of flow of positive charge) is from copper to zinc. In the present case, there is no flow of positive
charge as such from one electrode to another. But we can look upon the arrival of electrons on a copper
plate (with a subsequent decrease in its positive charge) as equivalent to an actual departure of positive charge from it.
When zinc is negatively charged, it is said to be at a negative potential with respect to the electrolyte,
whereas anode is said to be at positive potential relative to the electrolyte. Between themselves,
the copper plate is assumed to be at a higher potential than the zinc rod. The difference in potential is
continuously maintained by the chemical action going on in the cell which supplies energy to establish
this potential difference.